Does your child have uncontrolled hives?
Does your child have uncontrolled hives?
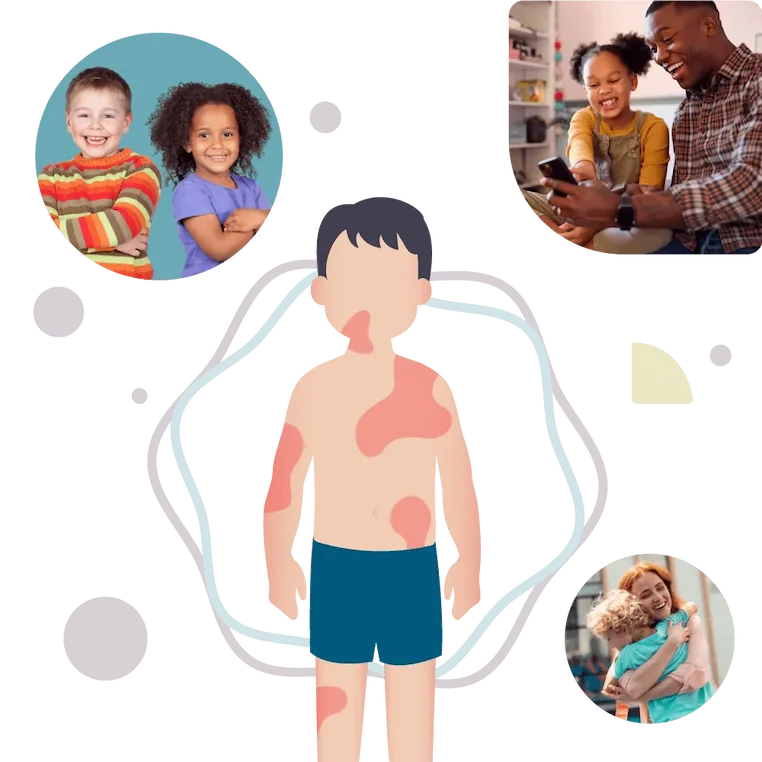
There is a new clinical trial for a medicine to safely and effectively treat hives, also known as chronic spontaneous urticaria (CSU).
If your child is between the ages of 2 and 17 and suffers from itchy hives, this study may interest you.
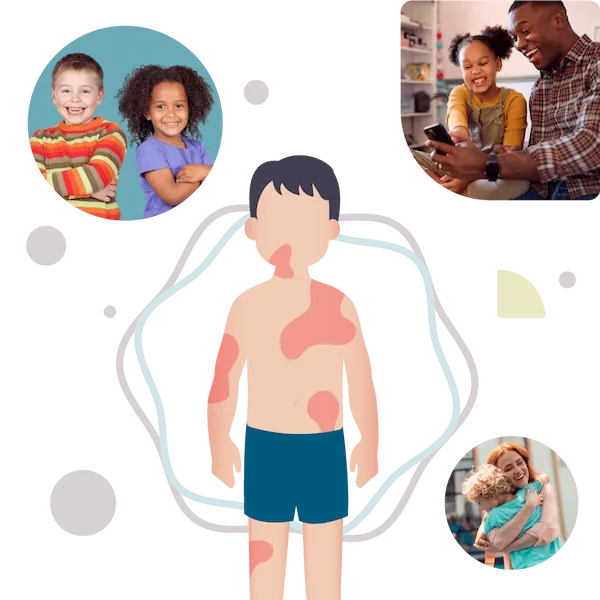
There is a new clinical trial for a medicine to safely and effectively treat hives, also known as chronic spontaneous urticaria (CSU).
If your child is between the ages of 2 and 17 and is suffering from itchy hives, this study may interest you.
About The Trial
Meet Our Medical Director
Dr. Mercedes E. Gonzalez is a board-certified pediatric dermatologist. After graduating from Emory University, she earned her degree at Rutgers–New Jersey Medical School in 2004. Always drawn to working with children, she accepted the prestigious pediatrics program at the Morgan Stanley Children’s Hospital of New York–Columbia University where she solidified her interest in treating skin disorders. She then completed a dermatology residency followed by a clinical fellowship in pediatric dermatology at the top-ranked New York University (NYU) Department of Dermatology.
Her gentle, child-friendly bedside manner, combined with her broad knowledge of childhood skin diseases and their treatments, make her the preferred pediatric dermatologist in Miami. In addition to practicing medicine, Dr. Gonzalez currently serves as a clinical assistant professor at The FIU Herbert Wertheim School of Medicine and The Phillip Frost Department of Dermatology at Miller School of Medicine.
Dr. Gonzalez serves as the Principal Investigator on numerous clinical trials and has a special interest in severe skin disease in children. She lectures regularly at Dermatology conferences and to medical students and residents and is the co-editor of 3 dermatology textbooks, including the recently published 2nd edition of Goodheart’s Same Site Differential Diagnosis, and has published over 50 journal articles.

Join Our Free Webinar
New Treatments For Hives In Children
📅Date to Be Determined
“Children are our most valuable resource.”
“Children are our most valuable resource.”
– Herbert Hoover


